
Organizations have risk management plans which are designed after careful consideration of numerous factors such as potential hazards and their probability of occurrence, the magnitude of the harm that could be caused by the hazards, or if the risk level reaches an unacceptable level.
#CAPA PROCESSES ISO#
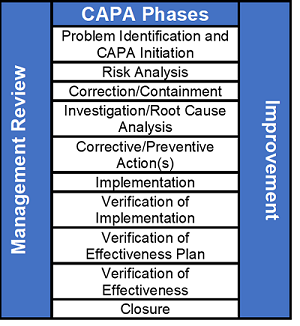
ISO 1341 provide guidelines for the quality processes centered around risk management of the processes.

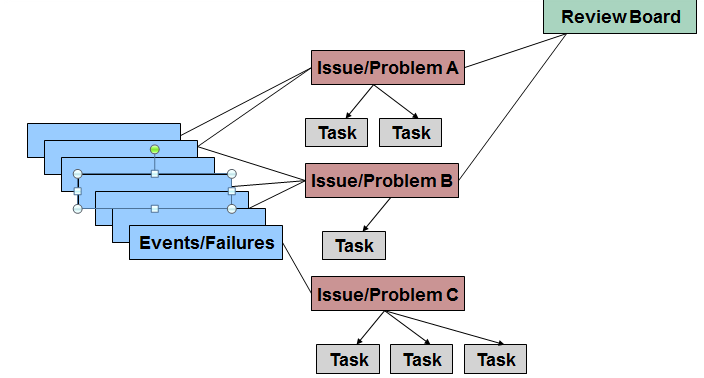
“Which non-conformance is related to this CAPA?”.This ensures that whenever you look at a CAPA, you can instantly find answers to questions like Paper-based processes might make it difficult to have this view but digital QMS allows you to link your CAPAs to the related items in the process. Tracking every minute detail ensures that you can catch patterns and gaps proactively rather than after the damage is done. Trace your CAPAs from their source to the resolution. Lack of traceability of updates to the ownership of CAPA.ĬAPA Process Improvements 1. Resource ownership of the CAPA – Advanced planning of who should own a CAPA and take it through to completion.Having the visibility of an issue’s prior occurrence/nonoccurrence could speed up the resolution process in some cases. Overlap and duplication issues leading to redundant work – Not knowing if a similar issue has occurred before either as a non-conformance, complaint or even a CAPA is a void that needs to be addressed.Lack of clarity on whether an issue should be treated as a CAPA or not – The research found that more than 70% of the CAPAs reviewed could be managed through existing quality systems and did not require to go through the CAPA Process.
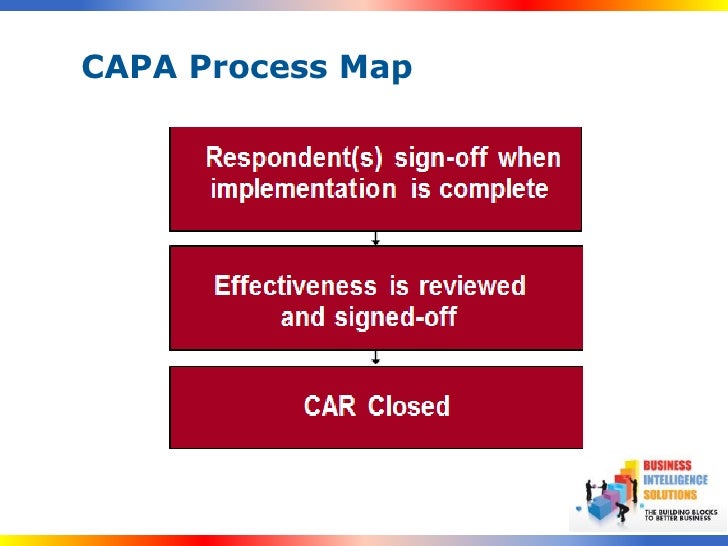
MDIC Case for Quality initiative conducted in-depth research and released the top 3 pain points that were put forth by people handling quality processes. “Our CAPA system is bloated and is focused on creating documents instead of resolving issues” It is burdensome and is seen as a punishment instead of an improvement opportunity” In MDIC Case for a quality program, organizations highlighted the mindset that has been built over the years due to the regulatory burden. Corrective Action and Preventive action is in fact an opportunity for improvements in the quality processes. One of the most talked-about and feared issues in a quality process is the CAPA. Often during this process, the regulatory burden makes it difficult for quality process individuals to provide their complete focus to solving the errors in a way that they are traceable backward, permanently fixed, and provide insights about any matters that need to be addressed immediately. The focus of these processes is to address the errors, analyze them, fix them and ensure that they do not occur again. To adhere with industry regulations and to remain compliant with various policies, quality processes of organizations have multiple checks and internal policies in place to address the non-compliances and errors that occur during the lifecycle of manufacturing a product. The practice must be to refine it and bring the errors down as low as humanly possible. There is always a percentage of error that happens. Errors are usually a part of any process, action, or activity.


 0 kommentar(er)
0 kommentar(er)
